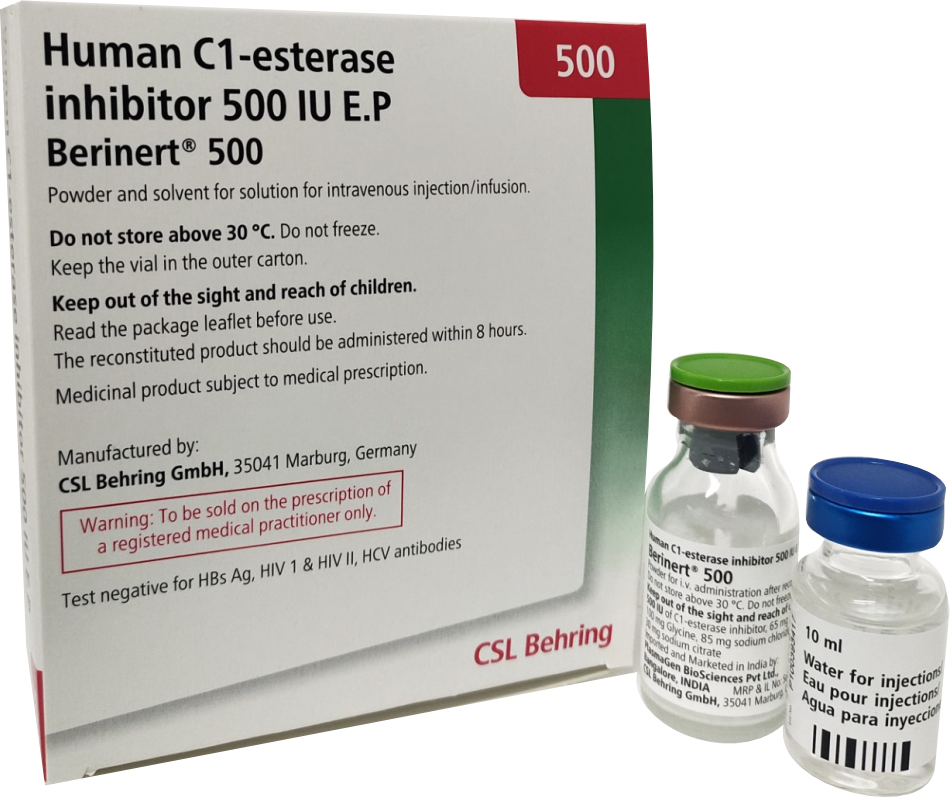
Product Description
Berinert 500 : Presented as a powder and solvent for solution for injection/infusion.
Indications
- Hereditary angioedema type I and II (HAE). Treatment and pre-procedure prevention of acute episodes.
Storage
- Do not store above 30 C.
- Do not freeze.
- Keep the vial in the outer carton, in order to protect from light.
For storage conditions after reconstitution of the medicinal product
After reconstitution the physico-chemical stability of Berinert 500 has been demonstrated for 48 hours at room temperature (max. 30 °C). For Berinert 1500, the physico-chemical stability has been demonstrated for 48 hours at room temperature (max. 25 °C). From a microbiological point of view and as Berinert contains no preservative, the reconstituted product should be used immediately. If it is not administered immediately, storage shall not exceed 8 hours at room temperature. The reconstituted product should only be stored in the vial.
Shelf-life
36 months from the date of manufacture
Virus safety
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses.
1. India approved prescribing information for Berinert ® , February 2018 Medical code:IND-BRN-0012